The secret to making batteries last longer could be in your fridge
A battery's winter home: between the ketchup and the relish
 Credit:
Reviewed.com / Julia MacDougall
Credit:
Reviewed.com / Julia MacDougall
Products are chosen independently by our editors. Purchases made through our links may earn us a commission.
Have you ever heard that old wives’ tale—that keeping batteries cold extends their lifespan?
I hadn’t, but then again, as a Millennial it had never occurred to me to look for ways to extend the shelf-life of AA batteries. I guess I’m just used to the “they just don’t make them like they used to” school of thought I’ve heard so often from people in my parents’ generation: I expect most things I buy to bite the dust within a couple of years, if not less.
Apparently, though, there's some truth to this old-timey hack. Let me explain.
Tell me how a battery works.
Alkaline batteries (aka your basic AA batteries) are made up of three components: positively charged material (the “cathode”), negatively charged material (the “anode”), and the fuel (“electrolyte”).
The fuel reacts with the anode material to create electrons that, when plugged into a circuit, flow through the wires from the anode (negative) terminal to the cathode (positive) terminal, and create electricity.
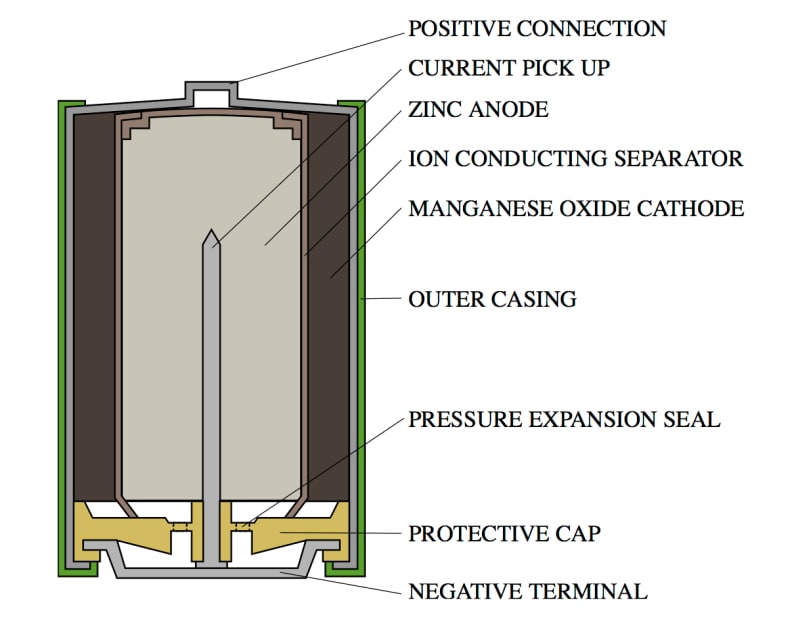
A cross-section of an alkaline battery.
In the case of AA batteries, the anode material is usually a zinc (Zn) compound, the cathode is a manganese dioxide (MnO2) compound, and the electrolyte is a paste or liquid containing potassium hydroxide (KOH) and some amount of water (H2O).
Batteries are built such that the zinc and the electrolyte are in contact with one another, but are physically separated from the manganese dioxide. The zinc and electrolyte are constantly undergoing an oxidation reaction to create negatively-charged electrons. These electrons congregate on a collection pin that is connected to the negative terminal of the battery.
Because the electrons are negatively-charged, they are attracted to positively-charged material, namely the manganese dioxide located on the positive end of the battery. When you plug a battery into a device, the circuit allows the electrons to flow through the device's circuitry to reach the positive end of the battery.
That constant flow of attracted electrons—from the negative to the positive end of the battery—is what we harness in the form of electricity that powers our remote controls, cameras, and other devices.
Okay, that makes sense. So what would refrigerating the batteries do?
During the chemical reactions described above, the electrolyte (KOH and water) not only provides fuel for the zinc oxidation reaction, it also provides a pathway for electrons to move from the zinc to the collection pin.
So basically, the electrolyte substance has water in it, and water—as we all know—can be frozen. By putting batteries in the refrigerator, you are slowing down the electrolyte’s ability to act as a medium through which electrons migrate from the zinc/electrolyte mixture to the collection pin.

Because the zinc and the electrolyte are in contact, the oxidation reaction is occurring constantly. However, that process is sped up when a battery is plugged in, as the electrons have easier access to the positively-charged battery terminal to which they are attracted.
When a battery is not plugged in, however, electrons are still being generated in a process called “self-discharge”. Over a long enough time span (we’re talking years, here), batteries can self-discharge enough so that they are no longer usable.
Refrigerating batteries is a way to help reduce self-discharge. It would be difficult to stop self-discharge altogether, but lowering the air temperature around the batteries should mitigate it to some extent.

Battery life, over a series of years, at three separate temperatures.
However, here’s the subtlety: for the same reasons that refrigerating a battery may help to elongate its lifespan while it’s not in use, it also makes it a lot more difficult for a battery to create that flow of electricity if you actually use the battery in cold temperatures.
Using batteries in cold temperatures inhibits the battery’s ability to transport electrons; as a result, you are decreasing the actual voltage you get out of the battery, and your device may not work as well.
Energizer, the battery manufacturer, has unsurprisingly done a lot of research on this topic, and their data supports the idea that batteries are less effective when they are used at refrigerator/freezer-level temperatures:

AA battery output voltage at three different temperatures.
Batteries, like human beings, operate most effectively at room temperature.
On the other hand, this Snopes article has quotes from Duracell and Energizer saying that batteries should not be stored at cold temperatures. However, this could be referring to short term storage, rather than long term storage.
Exposing batteries to cycling hot and cold temperatures can cause battery failure; the constant expansion or contraction of the individual components may break the seal on the battery and may result in leakage. For long-term storage (years, rather than days) prior to battery usage, a refrigerator may help to prolong battery life.
What’s the TL;DR?
- Long-term storage of batteries in the refrigerator (when they’re not in use/plugged in) may help to expand their usable lifespan.
- Avoid using batteries in cold temperatures as much as possible.
